FDA Clearance Granted to 4DMedical's CT:VQ
- Posted on September 4, 2025
- By Google News
- 5 Views
FDA Clearance Granted to 4DMedical's CT:VQ

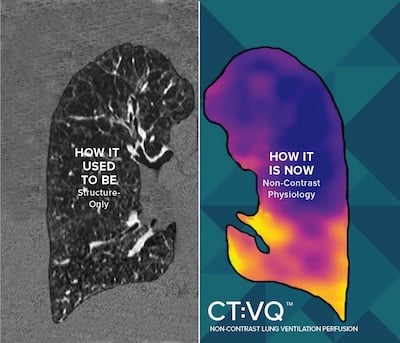
Sept. 4, 2025 — 4DMedical, a global medical technology company, has announced U.S. Food and Drug Administration (FDA) 510(k) clearance for CT:VQ, the world's the company's non-contrast, ventilation–perfusion (V/Q) imaging solution. In parallel, the U.S. Centers for Medicare & Medicaid Services (CMS) has confirmed reimbursement for CT:VQ under Category III CPT codes; this payment is in addition to existing reimbursement for the underlying chest CT.




